
Cannabis and UK Law
Under UK law, Cannabis is a Class B controlled drug under Part II, Schedule 2, of the Misuse of Drugs Act 1971 (MDA 1971). Drugs in schedule 2 can be prescribed and supplied by pharmacists and doctors, and as such, be possessed lawfully by anyone who has a prescription for specific medication. It is also listed in Schedule 1 of the Misuse of Drugs Regulations 2001 (MDR 2001) and designated under the Misuse of Drugs (Designation) (England, Wales and Scotland) Order 2015 (2015 Order); 1. meaning that possession of cannabis or cannabis resin is illegal without a license.
Cannabis is a controlled substance because it contains THC (tetrahydrocannabinol) and CBN (cannabinol), which have intoxicating properties and give the user the ‘high’ that most people associate with cannabis use. Growing or possessing cannabis plants legally, requires a Home Office license. Currently, only 2 licenses have been granted in the UK, which allow GW Pharmaceuticals and Northern Leaf to cultivate medicinal cannabis.
CBD and UK Law
CBD is not classed as a controlled substance under UK law as it does not cause intoxicating effects. Instead, CBD is classed as a novel food substance, and companies who produce and sell CBD extracts in the UK are required to comply with the Food Standards Authority (FSA) novel food regulations. From March 2021, any company extracting CBD for the UK market needs to have had their products authorised and validated to remain on sale.
Once initially authorised, products must be validated through the 2nd stage of the regulation process. This requires companies to submit toxicology evidence to ensure the safety of the product. The FSA has concerns about the safety of CBD. 2. As such, each novel food application is required to be submitted along with details of the toxicological studies they have undertaken, or propose to undertake for each individual product. Literature-based information is not sufficient and each application must include toxicology safety study data.
Novel Food Regulations will change the UK CBD Market
These new regulatory processes are expected to be expensive and are likely to result in a change in the number and variety of products available on the UK market. However, it will hopefully result in the demise of poor quality, inaccurately labelled products and as such, increase consumer confidence. It seems that it won’t be plain sailing. Although the majority of the 750 UK CBD brands currently in the marketplace are understood to be in the process of securing compliance, some are refusing, stating that whole plant extracts are not novel foods as they were in existence prior to the 1997 cut-off date.

Precautionary Control
CBD as an isolated substance, in its pure form, would not be controlled under the MDA 1971 / MDR 2001 in UK law. However, the Home Office states that in their experience, many products claiming to be pure CBD “do not fully disclose their contents or provide a full spectrum analysis at an appropriate level of sensitivity to accurately and consistently determine their true content or control status”. 1. Consequently, any product containing CBD is controlled as a precautionary measure because it is likely to also contain THC and/or CBN.
To ensure that CBD products do not contain excess prohibited cannabinoids, new guidance on the analytical limits for controlled cannabinoids was released in January 2021. 3. This states that there are twelve prohibited psychoactive cannabinoid compounds that occur naturally in cannabis plants, which could be present in CBD extracts or isolate. In order to analyse the quantity of these compounds effectively, these twelve cannabinoids have been grouped together, as shown in Table 1.
| Group | # | Name |
| A | 1-3, 5 | Delta-9-tetrahydrocannabinol (Δ9-THC) |
| B | 4 | Delta-9-tetrahydrocannabivarin (Δ9-THCV) |
| C | 6 | Delta-8-tetrahydrocannabinol (Δ8-THC) |
| D | 7-11 | Cannabinol (CBN) |
| E | 12 | Cannabinol methyl ether-C5 |
Table 1: Prohibited intoxicating cannabinoid compounds classified by group
Legal THC Percentage Concentration
Although both THC and CBN cannabinoid compounds are controlled under UK law, THC is the best known and most talked about. There seems to be some confusion over the concentration of THC that is legally permitted in CBD products. During the extraction process, all cannabinoids present in the plant material are extracted, not just CBD alone. Completely removing THC (and/or CBN) is a time-consuming, complex process, so a small quantity is permitted in the end product.
This is also beneficial to the user through a phenomenon called the “Entourage Effect”, where the blend of different cannabinoids, alongside other chemicals in the plant called terpenes and flavonoids, synergistically work together in a way that’s superior to the way the individual molecules work alone. 4. Perhaps more confusingly, these concentrations differ, dependent on where in the world you live. The USA, and more recently Europe, allow 0.3% THC while the UK currently only allow 0.2%. But what does that actually mean?
0.2% THC Reference
In UK law, the 0.2% THC reference that is regularly bandied around, is actually used solely to identify varieties of cannabis plants with a low THC content. These strains are used for the production of hemp fibre for industrial purposes (including CBD production), or obtaining seeds which are then pressed for their oil. It should be noted that this is only applicable to the non-controlled parts of the plant (i.e. seeds and fibre/mature stalk only). 1. In the UK, it is illegal to use the flower and buds for any purpose. The flowers and buds from hemp are classed as ‘cannabis’ and are controlled under the MDA even if they are an authorised strain that contains less than 0.2% THC.
Hemp Flowers and Buds
You can’t tell by just looking at the flowers or buds whether they are from low THC hemp or high THC cannabis plants. The flowers and buds, which contain the highest concentration of cannabinoids in the plant, must be destroyed on site as it is illegal to transport them. Despite this, some UK companies currently sell CBD flower under the guise of CBD tea or similar products. This may change with the introduction of the novel food regulations.
Only lab analysis from a reputable lab that has the ability to measure even trace amounts of THC can determine the quantity of THC and other prohibited cannabinoids present in these products. 3rd party, independent lab analysis is the gold standard in the CBD industry. UK hemp stakeholders recently published a manifesto calling for the government to deschedule cannabinoids and set a 1.0% THC standard to develop a vibrant hemp sector in post-Brexit Britain.
Legal THC Content in CBD End Products
Different criteria apply to end products containing CBD and other cannabinoids. For the product to be considered exempt from control under UK law, it must not contain any more than 1 milligram (mg) of a controlled drug. It is the Home Office view that the applicable unit of measure for the 1mg ‘threshold’ referred to is that of the ‘container’ (i.e. bottle or packet) and not the ‘typical dose’ (of any product). 1.
The ‘controlled drug’ may not consist of a single compound but could include all twelve prohibited cannabinoid compounds that when added together may exceed the one milligram threshold. As such, each prohibited cannabinoid compound requires detection at a lower concentration. Each prohibited cannabinoid compound should therefore not exceed 0.0833mg per mg of extract (if present in equal amounts). 3.
1mg THC/CBN per Container Threshold
In simplistic terms, to be exempt from control, there cannot be more than 1mg of combined THC or CBN compounds (combined controlled drug) present in any individual container of finished CBD product regardless of size. The 0.2% THC level does not apply to finished products. If it did, the 1mg limit at a 0.2% percentage concentration would occur in a container of 0.5g or 0.5ml of product. As the smallest container is unlikely to contain less than 1g or 1 ml of product, these would only have a THC percentage concentration of 0.1% at the maximum 1mg dose of controlled drug.
Typically, CBD product containers range between 5ml and 200ml, and as the volume increases, the percentage concentration of controlled cannabinoid decreases (assuming the product contains the full 1mg dose of combined controlled drug), as shown in Table 1. 3. This means that if a product in any container greater than 0.5ml states that it contains 0.2% THC, the THC dose will be greater than 1mg, and as such, illegal.
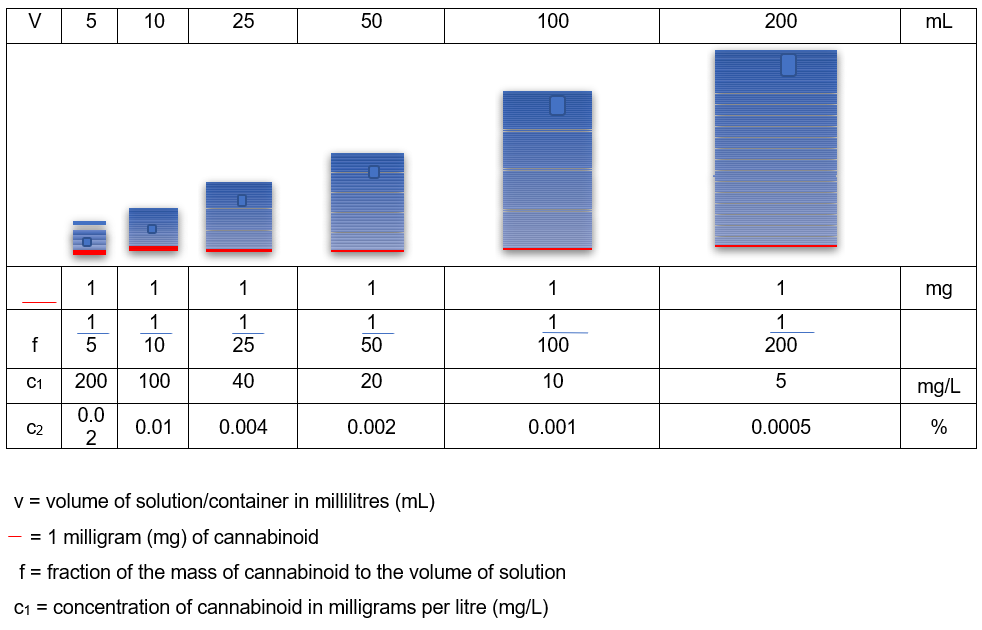
Table 1: Concentration of 1mg controlled cannabinoid with increasing volume (from 3.).
Changes to Legislation
Changes are starting to happen with regards to both global and UK legislation. In the global context, under the recommendation from the World Health Organisation (WHO), the United Nations Commission on Narcotic Drugs (CND) voted to remove cannabis and cannabis resin from Schedule IV of the 1961 Single Convention on Narcotic Drugs in December 2020. Demonstrating that cannabis has been recognised globally as having medicinal value.
Medicinal Benefits of Cannabis Acknowledged
The therapeutic and medicinal benefits of Cannabis based products were acknowledged in the UK in 2018 when cannabis for medicinal purposes was reclassified as a Schedule 2 drug under the Misuse of Drugs Act. Allowing medicinal cannabis to be prescribed for specific conditions, such as Multiple Sclerosis and chronic pain.
In June 2020, GW Pharmaceuticals’ cannabis-based drug Epidyolex was moved from Schedule 2 to Schedule 5. This move was made to make the drug easier to prescribe and dispense to patients. It’s my own personal view that this was granted so easily due to the drug only containing purified cannabidiol (CBD), which technically is not a controlled substance and GW Pharma were able to prove the purity of the isolate. However, it begs the question, will only large pharmaceutical companies be granted such permissions? It was only their licensed medicinal product that was reclassified and not purified CBD products as a whole.
Changes to European CBD Regulation
Changes have also occurred across the European Union (EU) where CBD’s original designation of a novel food was challenged by a number of member states. Individual countries, including France, tried to have CBD reclassified as a narcotic and banned its sale. However, in November 2020, the European Court of Justice over-ruled the EU’s preliminary stance, making it clear that CBD extract from flowers is not a narcotic and that member states cannot prohibit the marketing of CBD legally produced in another member state when it is extracted from the cannabis sativa plant, including flowers. They also insisted that France must provide scientific proof of the dangers of CBD to keep their ban on the marketing of CBD-infused products in place.
Changes to UK CBD Regulation
The UK followed the EU directives in designating CBD as a novel food, with the Food Standards Authority (FSA) regulating CBD products. No longer being part of the EU, the UK were able to reject the EU’s stance that hemp flower-derived CBD should be regulated as a narcotic, and do not have to follow the subsequent EU ruling, although already agreeing with it. However, as previously stated above, in the UK it is illegal to extract CBD from the flowers of any cannabis or hemp plant regardless of THC/CBN concentration.
It is unclear whether CBD extract from flowers will remain illegal or if it could be authorised for use in CBD products through the FSA’s novel food regulations. Flowers and buds being the main production sites of CBD and other cannabinoids, produce the highest yield of extracts. As such, UK CBD extraction and production companies hope to see flowers and buds from authorised low THC strains of Cannabis sativa plants being removed from the MDA to allow their use.
NEXT: “Pets: Is it legal to give them CBD?”
References
1. Home Office 2019. Factsheet- Cannabis, CBD and other cannabinoids. Available at https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/825872/factsheet-cannabis-cbd-and-cannabinoids-2019.pdf
2. Committee on Toxicity, 2020. Position paper on the potential risk of CBD in CBD food products. Available at https://cot.food.gov.uk/sites/default/files/2020-08/cbdpositionpaper290720_accessibleinadobepro.pdf
3. Walker, M. and Axford, I. Government Chemist Guidance: Analytical Limits for Controlled Cannabinoids in Specified Products Containing Cannabidiol (CBD) Available at https://www.gov.uk/government/publications/guidance-on-analytical-limits-for-controlled-cannabinoids
4. Koltai, H. and Namdar, D., 2020. Cannabis Phytomolecule ‘Entourage’: From Domestication to Medical Use. Trends in Plant Science.
5. Professor Dame Sally Davies (CMO), 2018. Cannabis Scheduling Review Part 1: The therapeutic and medicinal benefits of Cannabis based products – a review of recent evidence. Accessed via https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/722010/CMO_Report_Cannabis_Products_Web_Accessible.pdf

